Protocols
Flow cytometry
Protocol created by Lorena Maestre - Monoclonal Antibodies Unit, Centro Nacional de Investigaciones Oncológicas
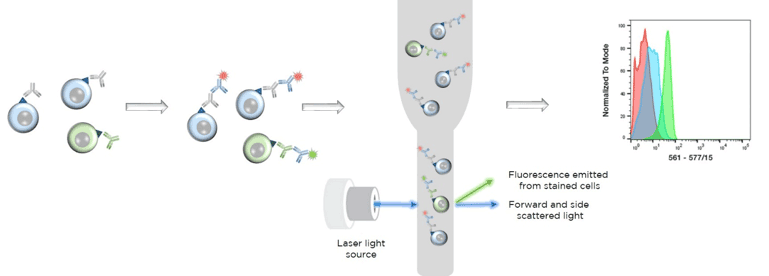
Flow cytometry sample preparation for surface antigens
Material
Phosphate buffered saline (PBS) - 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4
Bovine serum albumin (BSA, Biolabs)
Ethylenediaminetetraacetic acid (EDTA, Merck)
Fc receptor block - e.g. human serum or Trustain (Biolegend) for human cell samples, anti-mouse CD16/CD32 antibody (clone 2.4 G2, Stemcell Technologies, BD Biosciences) or non-immune mouse serum for murine samples.
Primary antibody - unlabelled or conjugated with a fluorescent tag.
Fluorescent conjugated secondary species-specific antibody (if required) - e.g. Alexa fluor 488 goat anti-mouse (Invitrogen), Alexa fluor 488 chicken anti-rat (Life technologies) or donkey anti rabbit IgG Alexa fluor 488 (Thermo-Scientific).
Dead cell exclusion dye - Either i) DNA binding dyes e.g. propidium iodide (PI), 4’, 6’-diamidino-2-phenylindole, dihydrochloride (DAPI), 7-amino-actinomycin D (7-AAD, Thermo Scientific) or ii) amine-reactive fixable viability dyes e.g. ViViD or Aqua Blue (Invitrogen)
CellTrics filters (Sysmex) or any other cell filter.
Method
Before starting, remember that it is very important to prepare the appropriate control samples in order to ensure accurate flow cytometry signals. Negative controls are unstained cells alone and cells stained only with the secondary antibody (if used). Working dilutions of Fc receptor blocker should be identified. Primary and any secondary antibodies should be titrated to obtain their optimal working dilutions.
Perform all the staining on ice to prevent downregulation of surface markers due to regular cellular metabolism or any non-specific binding of antibodies.
- Harvest cells, wash once in PBS at 500g for 5 min.
- Resuspend cells in PBS to a concentration raging from 60 x 106/ml for lymphocytes and 10x106/ml for larger cell, such as fibroblasts. Larger cells have a tendency to clump and should therefore be used at lower concentrations.
- Take 50μl (3x106 cells or the desired quantity) and place into an Eppendorf tube
- Centrifuge 500g for 5 min, remove the supernatant by decanting or using a vacuum pump.
- Resuspend the cells in a final volume of 50μl of Fc receptor blocking buffer (e.g. Trustain 2.5μl per 50μl PBS) for 5-10 min.
- Wash once in PBS and centrifuge at 500g for 5 min, remove the supernatant. It is possible to skip this wash and just add your primary antibody directly to the Fc receptor block.
- Add primary antibody to give a final volume of 100ul. (For supernatants use 10-20ul/tube and for purified antibodies use 1:200-1:400 dilutions), incubate for 30 min in the dark if a fluorescent conjugated primary antibody is used.
- Wash once in PBS at 500g for 5 min, remove the supernatant
- If required add 100μl of a species-specific conjugated secondary antibody (1:200-1:600 dilution in PBS) and incubate for 20min in the dark.
- Wash once in PBS and centrifuge at 500g for 5 min, remove the supernatant.
- Resuspend into 300μl of PBS/ 0.1% BSA/3mM EDTA or PBS only.
- Add 2μl of DAPI per tube.
- Filter the cells using Celltrics filters to eliminate aggregates.
- Bring the tubes to the flow cytometry equipment and stablish the settings by using the positive and negative controls.
Flow cytometry sample preparation for intracellular antigens
Materials
Phosphate buffered saline (PBS) - 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2HPO4
Bovine serum albumin (BSA, Biolabs)
Ethylenediaminetetraacetic acid (EDTA, Merck)
Saponin (Merck)
Triton X-100 (Merck)
Methanol (Merck)
Aqua LIVE/DEAD Fixable (Invitrogen) or any fixable viability dye
Paraformaldehyde (PFA, PanReac AppliChem)
Fc receptor block - e.g. human serum or Trustain (Biolegend) for human samples, anti-mouse CD16/CD32 antibody (clone 2.4 G2, Stemcell Technologies, BD Biosciences) or normal non-immune mouse serum for murine samples.
Primary antibody – unlabelled or conjugated with a fluorescent tag.
Secondary fluorescent conjugated antibody (if required). There are several companies that sell secondary antibodies suitable for flow cytometry, e.g. Alexa fluor 488 goat anti-mouse (Invitrogen), Alexa fluor 488 chicken anti-rat (Life Technologies) or donkey anti-rabbit IgG Alexa fluor 488 (Thermo-Scientific).
CellTrics filters (Sysmex) or any another cell filter
Method
Before starting, remember that it is very important to prepare the appropriate control samples in order to ensure accurate flow cytometry results. Negative controls are unstained cells alone and cells stained only with the secondary antibody (if used). Working dilutions of the Fc receptor blocker should be identified. Primary and secondary antibodies should be titrated to obtain their optimal working dilutions.
The PFA fixation step should be carried out at room temperature. All other staining steps should be performed on ice to prevent downregulation of surface markers due to regular cellular metabolism and to minimize any non-specific binding of antibodies
- Harvest cells, wash once in PBS at 500g for 5min.
- Resuspend cells in PBS to a concentration ranging from 60x102 cells/ml for lymphocytes to 10x102 cells/ml for larger cells, such as cultured fibroblasts. Larger cells tend to clump and should therefore be used at lower concentrations.
- Take 50μl (containing 0.5 to 3x102 cells depending on cell type) and place into each Eppendorf tube
- Add 1μl per 1x102cells of the viability marker Aqua LIVE/DEAD Fixable and incubate for 30 min.
- Centrifuge at 500g for 5 min and remove the supernatant.
- Fix the cells in 2% PFA for 15 min at room temperature. Be aware that fixation can change epitopes so be sure that your primary antibody works on fixed antigens. An alternative is to stain LIVE cells, fix the cells and then permeabilize them to label intracellular antigens.
- Wash twice with PBS/0.01% Triton. Centrifuge at 500g for 5 min and remove the supernatant.
- Permeabilize with PBS/1% Triton for 20 min.
- Wash twice with PBS/0.01% Triton. Centrifuge at 500g for 5 min and remove the supernatant
- Resuspend in 50μl of Fc receptor blocking buffer (Trustain 5μl/100μl PBS) for 5-10min.
- Wash with PBS/0.01% Triton or 0.1% saponin in PBS (both make transient holes. Use ice-cold methanol if permanent holes are required). Centrifuge at 500g for 5 min and remove the supernatant
- Add primary antibody to give a final volume of 100μl in PBS/0.01% Triton and incubate for 30 min in the dark if a fluorescent conjugated primary antibody is used. For supernatants use 10-20μl/tube and for purified antibodies use 1:200-1:400 dilutions.
- Wash once in PBS/0.01% Triton and centrifuge for 500g for 5 min and remove the supernatant.
- If required add 100μl of a species-specific conjugated secondary antibody (1:200-1:600 dilution) and incubate for 20 min in the dark.
- Wash once in PBS/0.01% Triton and centrifuge at 500g for 5 min, remove the supernatant.
- Resuspend into 300μl of PBS/0.1% BSA/3mM EDTA or PBS only.
- Filter the cells using Celltrics filters to eliminate aggregates. DO NOT ADD DAPI.
- Bring the tubes to the flow cytometry equipment and stablish the settings by using the positive and negative controls.
